UDI Certificate Types
Every medical device sold in Europe requires a valid approval certificate in accordance with MDR 2017/745 and IVDR 2017/746. It certifies that the product meets all regulatory requirements of a medical device. By combining a product group into a Basic UDI-DI, only one certificate is required for a product group instead of for each item within a product group. The certificate type specifies the type of the product certificate associated with the device.
UDI certificate types are currently not relevant for the FDA.
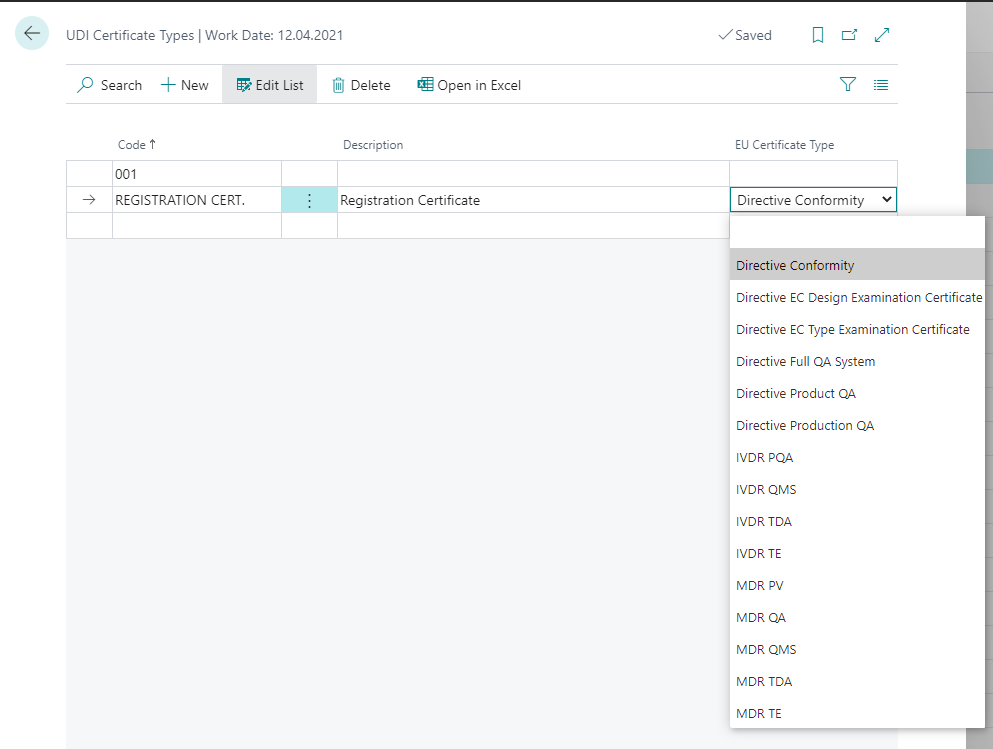
| Field | Description |
|---|---|
| Code | Specifies a certificate type code that you can select. |
| Description | Specifies a text to describe the certificate type. |
| EU Certificate Type | Specifies the certificate type that will be used when exporting to EUDAMED. |
Feedback
Submit feedback for this page .