UDI Notified Bodies
Every medical device sold in Europe will require a valid approval certificate in accordance with MDR and IVDR. This certification is carried out by a notified body. This notified body must be assigned to the respective certificate. The notified bodies can be stored as master data.
The UDI Notified Bodies page is accessed using Tell Me. Choose the  icon, enter UDI Notified Bodies, and then choose the related link.
icon, enter UDI Notified Bodies, and then choose the related link.
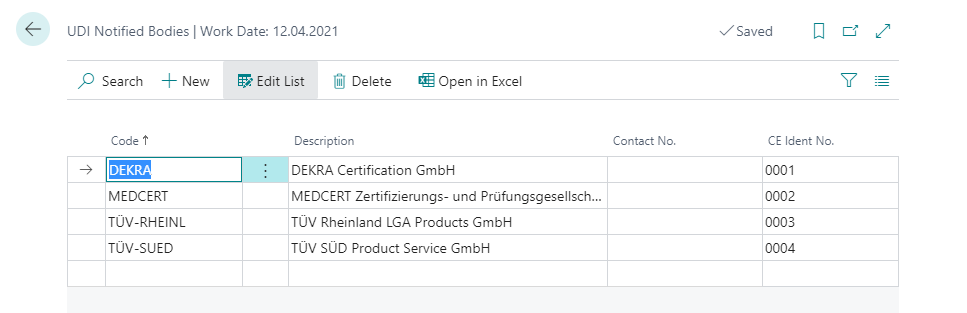
| Field | Description |
|---|---|
| Code | Specifies a notified body code that you can select. |
| Description | Specifies a text to describe the notified body. |
| Contact No. | Specifies the number of the contact that provides further data such as the address. |
| CE Ident No. | Specifies the four-digit identification number required for printing CE marks for basic UDI-DI data. |
Feedback
Submit feedback for this page .